










 |
 |
 |
 |
 |
 |
||
 |
 |
 |
 |
 |
 |
ionising radiation
|
|||
|
|
|
|||
| Ionising radiation and health—risk analysis is the sixth of a series of briefing documents on the problems of power consumption, posed by the steady depletion of fossil fuels and most particularly of pumpable oil. |
| on energy | on global warming | |||
| sustainable futures briefing documents |
Tectonics: tectonic plates - floating on the surface of a cauldron |
|||
Food: eating is bad for you!The lifetime risk of cancer: |
||
from the food you eat |
6.6% |
|
from spices and flavourings |
less than 0.1% |
|
all other additives such as pesticides, drugs fed to farm animals and processes of food preparation |
less than 0.05% |
|
That is, the risk of eating at all is around 60 times the risks of all those additives that the media rattle on about. If you are really worried, perhaps you might like to starve! [1] Now that is just the cancer risk from eating that dangerous stuff called food. You might also choke on the stuff, get mad cow disease (BSE), or some nasty, potentially fatal, e-coli bug like E.c. 0157:H7. Even if you donít die from eating food, it is quite likely that you will still die! Be happy, donít worry Ė well, at least not so much. There is no such thing as a dangerous
substance or a poison, During radioactive decay, an unstable nucleus usually emits alpha particles, electrons, gamma rays, and neutrinos. In nuclear fission, the unstable nucleus breaks into fragments, which are themselves complex nuclei, along with other particles such as neutrons and protons. The resultant nuclear fragments are often in a highly excited state, and then reach their low-energy ground state by emitting one or more gamma rays. Because gamma rays have no electric charge, and thus do not interact with
matter as strongly as do charged particles, they have great penetrating power.
Because of their penetrating power, gamma rays can be used for radiographing
holes and defects in metal castings and other structural parts. At the same
time, this property makes gamma rays extremely hazardous. The lethal effect
of this form of ionising radiation makes it useful for sterilizing medical
supplies that cannot be sanitized by boiling, or for killing organisms that
cause food spoilage. More than 50 percent of the ionising radiation to which
humans are exposed comes from natural radon gas, which is an end-product of
the radioactive decay chain of natural radioactive substances in minerals.
Radon escapes from the ground and enters the environment in varying amounts. Naturally produced background radiation |
||
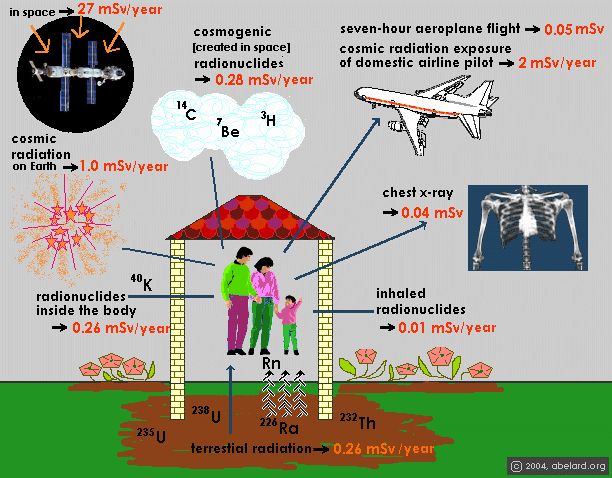
Naturally produced background radiation comes
from a number of sources, as shown in the illustration above. |
A comparison of the activities of selected radioactive materials
Note that plutonium is 153,000 times more active than depleted uranium. (2,298,000/15=153200) U238, U235 and U234 predominantly emit alpha particles (over 95% are alpha particles). The alpha activity of natural uranium amounts to about 25 kBq/g. The progeny from the alpha decay of uranium themselves continue to decay, mostly by emitting beta particles. The activity of these progeny is added to that of uranium. The beta radiation of the progeny of natural uranium and depleted uranium have practically the same intensity, amounting to about 25 kBq/g.
Uranium, together with its progeny, has an activity of 50 kBq/g (for instance, 50 000 decays take place per gram per second). The very long half-life [read this linked section now, if you do not understand half-life] of U238 (4.5 billion years) yields a low decay rate per unit mass of uranium. Naturally occurring uranium, which mostly consists of U238, is one of the least radioactive substances containing unstable isotopes on the planet. It is classified by the International Atomic Energy Agency in the lowest hazard class for radioactive materials. Exposure to the radiation emitted from uranium can occur if it is outside the body, or if it is ingested, inhaled or taken in by other means. It is useful to consider the exposure pathways with regard to average radiation exposure in the normal environment. As you can see in the illustration, gamma radiation can travel right through you from the environment and alpha radiation can be stopped by your skin. However, should you ingest radionucleides (radioactive materials) and they become lodged in the body, the alpha radiation will become a considerably greater problem because the radionuclides will not just wash or fall off. Alpha rays are 20 times more effective than beta and gamma rays at causing tissue damage. To allow for this, the dose in grays is multiplied by an effectiveness factor and the new units are called sieverts (abbreviation Sv) and the dose is called the equivalent dose. The Sievert (Sv) is the international measure of radiation expressed as a dose-equivalent. In the general population ingestion of uranium and its decay series in food and drink gives a committed effective dose of 0.11 mSv per year for adults, as compared to 0.0058 mSv through inhalation, excluding inhalation of radon (1.2 mSv). This annual dose corresponds to 5% of the average annual dose due to internal
and external exposure to natural sources of radiation (2.4 mSv). It relates
essentially to progeny, Pb210 and Po210. U238 accounts only for 0.00025 mSv
total dose and 0.000021 mSv for inhalation. External exposure from all natural
U238 in soil is also negligible. U238 series, together
with other primordial radionuclides, Th232 and K40, cause a world-average
annual external exposure of about 1 mSv per year. |
RisksIf I have a 1 in 1 million chance of being in an aeroplane crash and my risk doubles, I have then a 2 in 1 million chance of being in a crash. These rates are worked out by calculating, for example, how many people on average died in such crashes over the last 10 years. If none were killed in 9 of the years and 20 in the final year, that will give you an average of 2 each year. Of course in the year that 6 die in a crash, the Daily Slime will Ďreportí as follows: ď200% more killed in ’plane crashes this year, something must be doneĒ. (2 is the 100% base average figure, 6 is three times 2 and is therefore 300% relative to the base rate, hence the rise of 200% above the base rate.) Although, knowing full well the ignorance of the reporters at the Daily Slime, they will probably say 300% because they canít count either. The chance of being killed on the roads of America† is approximately 1:8000 each year, over 80 years that is a risk of 1 in 100. This is now becoming a serious risk compared with figures for flying! A doubling of that road-kill risk would be serious news! The risk of being killed while flying on large commercial jets is around 1 in million, if you fly 200,000 miles a year (the risks are higher on commuter flights and higher still in a private aircraft). Clearly you will not be killed in an aircraft if you do not fly in one; but you can still worry if you like; there is even about 1 chance in 25 million that an aeroplane will fall on you sometime in your life! It is important to
It is further important to remember that
|
X-raysYou may find some comments and references on medical x-ray radiation here. X-rays are a type of penetrating radiation that, depending on the dose, can reduce cell division, damage genetic material, and harm unborn children. Exposure to x-rays is measured in units of radiation absorbed dose (rad). Cells that divide quickly are very sensitive to x-ray exposure. Unborn children are particularly sensitive to x-rays because their cells are rapidly dividing and developing into different types of tissue. Exposure of pregnant women to sufficient doses of x-rays could possibly result in birth defects or illnesses such as leukaemia later in life. With most x-ray procedures, relatively low levels of radiation are produced. However, a doctor may decide to postpone or modify abdominal or lower back x-rays in a pregnant woman unless absolutely necessary. Women who receive x-rays before realizing they are pregnant should speak to their doctors. Some pregnant women may be exposed to x-rays in the workplace, so governments may establish limits to protect unborn children from radiation exposure in work settings. The
rate of risk of cancers from X-ray exposure are calculated from atomic
bombs and other exposures, extrapolating for the smaller exposures given
by X-rays. |
High altitudes, including flightThere is some theoretical effect, but empiric results do not easily detect it.
depleted uranium
Aspects of depleted uranium munitions [.doc format] The person who wrote this document has a neat sense of humour. Obviously, they also have had to deal with moonbats!
|
Some definitions
|
Bibliography |
|
| Danger ahead—the risks you really face on life’s highway by Larry Laudan, 1997, Wiley & Sons, pbk, 0471134406 |
|
| The culture of fear—why Americans are
afraid of the wrong things by Barry Glassner, 1999, Basic Books, pbk, 0465014909 |
|
| Encyclopedia Britannica | |
End notes |
|
|
|
© abelard, 2004, 05 february the address for this document is https://www.abelard.org/briefings/ionising-radiation.php 3540 words |
| latest | abstracts | briefings | information | headlines | resources | interesting | about abelard | ||